
High Throughput Equipment at HTD
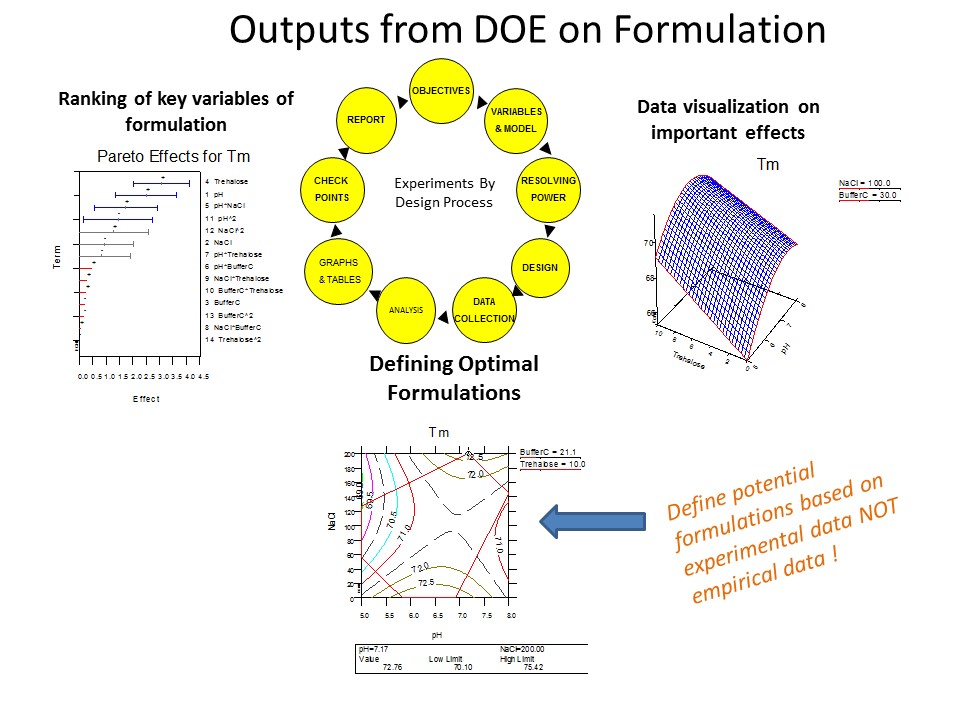
We are experts in DOE 2.0, where Formulations and Process are Rationalized.
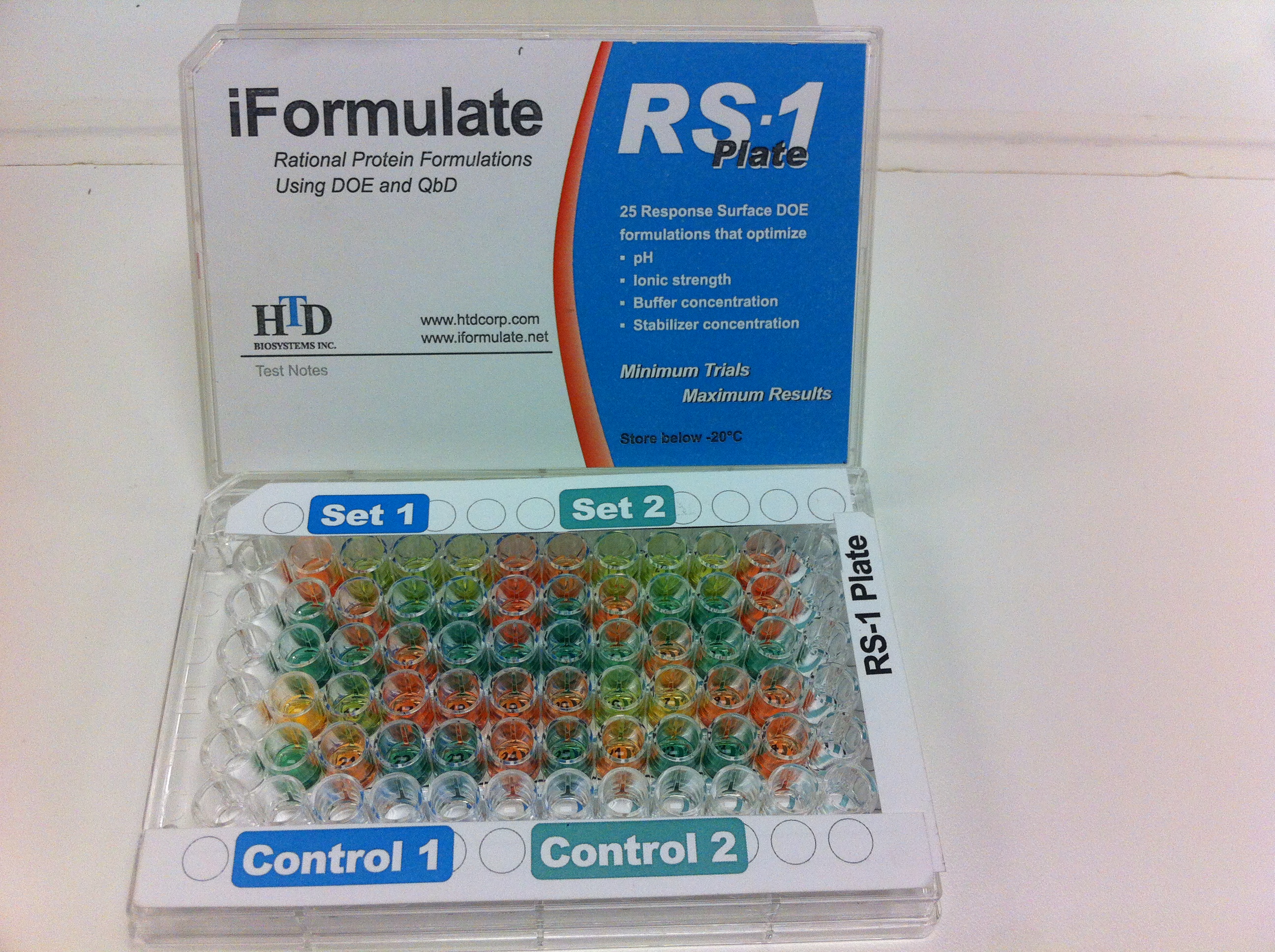
Our iFormulate technology can formulate a protein in an afternoon Precisely, Accurately, and Reproducibly! Visit www.iformulate.net

Customized lyophilization services and solid-state characterization.
Our Company
HTD Biosystems, is a contract formulation development lab that provides services for accelerating development of parenteral drugs, biosimilars and vaccines, using high throughput technologies. HTD’s expertise is in design of experiments (DOE 2.0), protein formulation, vaccine development, drug delivery systems, and lyophilization.
Our Story
In 2001, we envisioned what it would be like to be able to develop 10-20 or more projects every year because of the Genome Project and possibility of Research to generate many many exciting biotech drugs due to the Systems approach to biology. A blueprint was developed incorporating principles used in High Tech, Nanotechnology, Robotics, Computer algorithms, Program management tools, and many other advances in accelerating the workflow in technology and development.
The basic question we asked was how is it that Boeing, for example, can build one of the safest planes (Boeing 777) in 5 years from conception to production and we in the Pharmaceutical company take 10-15 years with a success rate of 5-20 % from Development to Product! One of the bottlenecks is CMC (chemistry, manufacturing, control), i.e ability to identify, make, and have quality of a potential Drug Product. According to the FDA, 5-7 out of the 10 drug products could be rejected because of CMC-related issues (wrong dosage form, instability, quality attributes contributing to safety, inconsistent manufacturing, etc).
HTD is a private company located in Pleasanton, California. HTD has worked and collaborated with other companies, governments and academia. Our researchers have helped other companies develop their products. An example of a product that was formulated and developed at HTD Biosystems for a Japanese biotech company, is a liposomal formulation against graft-versus-host disease (GvHD) associated with hematopoietic stem cell transplantation (HSCT). This product is now in Phase II clinical trials.
Our Approach (“Be nimble and collaborative”)
HTD Biosystems accelerates development of biotech drugs, using a unique formulation development platform iFormulate. This concept is based on an efficient high throughput algorithm that utilizes rational design of experiments and formulation parameters, to develop pharmaceutically acceptable drug product. Protein formulation development by iFormulate.
WE ARE NOT A ONE-STOP SHOP, BUT we could be your ONE-STOP SOLUTION because we believe it takes a “village to develop a great Drug Product”. Our village consists of our clients, our alliances, and many collaborators who are the world’s foremost experienced experts in what they do. Our network consists of the best researchers whose specialities help us address tough problems and issues and organizations who have extensive experience in bringing some of the top Drug Products to market. Our experience in working on many new and exciting products and technologies gives us a unique “out-of-box” prespective on addressing challenging issues in developing a well-characterized, safe, and efficacious Drug Product that can be manufactured consistently.
Our Accomplishments
HTD is a private company located in Pleasanton, California. HTD has worked and collaborated with other companies, governments and academia. Our researchers have helped other companies develop their products.
Since 2001, we have helped many clients with development of their protein drug candidate into a stable drug product. We have developed rational stable protein/peptide/small molecule formulations, developed robust and efficient lyophilization processes, novel drug delivery systems, novel vaccines, and well characterized the Drug Products using techniques that are Precise, Accurate, and Reproducible. We have solved many challenging formulation problems such as protein aggregation, solubility problems, establishing a scalable and well-characterized formulation process, and implementing state-of-the-art new technologies in characterizing the Drug Product.
Example 1: Using our iFormulate platform, HTD can perform rapid antibody screening and selection for the most stable formulation using less than 5 mg of protein in an afternoon. We are helping many small biotech companies evaluate their drug candidates from research from for conformational, thermal and colloidal stability utlizing 5-10 mg of precious material Faster, Better, and Cost-effectively.
Example 2: We took a complicated drug delivery product (a novel vaccine) that was formulated, scaled up to cGMP standards, manufacture of multiple Tox lots at HTD. Critical Process Parameters (CPP) and Quality Product Attributes were established in addition to novel assays (qualified and validated) for the Drug Product. Clinical lots of the Drug Product was successfully manufactured in cGMP facility. The complete development program was developed at HTD Biosystems with our Client and Collaborators for a Japanese biotech company. The Drug Product was a complex liposomal formulation against graft-versus-host disease (GvHD) associated with hematopoietic stem cell transplantation (HSCT). This product has successfully finished Phase II clinical trials. The Drug Product also was a stable liquid formulations with more than 2 years shelf life.
Example 3: We helped evaluate the Developability of antibody candidates for an emerging Biotech company using our High Throughput Technologies using limited amounts of available protein. Our recommendation was selected as the Lead candidate for development and the company is now a publicly traded company. We continue to do such projects for many biotech companies utilizing our High Throughput Systems Approach to Accelerate Development of exciting new biologicals.
Example 4: HTD was the Formulation Development partner in a 12 million dollar NIH/NIAID grant for the development of a multi-valent vaccine against Botulinum Neurotoxin. A pentavalent vaccine was successfully formulated into a Drug Product using 5 recombinant serotypes of Botulinum antigenic subnits in less than 3 years. This project was a collaboration between University of Nebraska, Lincoln, USAMRID, and DynPort Vaccine Co. HTD and University of Colorado (Carpenter and Randolph labs) collaborated heavily in basic vaccine research and characterization resulting in numerous publications.
Example 5: HTD has been involved in many Biosimilar projects (both liquid and lyophilized Drug Products). Projects have involved comparibility studies, biophysical characterization, formulation development, lyophilization development, and process development.
